Introduction
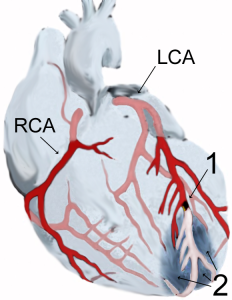
Myocardial infarction, with blockage (1) leading to tissue death (2) courtesy of J Heuser
The term acute coronary syndrome (ACS) describes three related conditions: ST elevation myocardial infarction, non-ST elevation myocardial infarction (NSTEMI), and unstable angina. All three share the common pathological process of insufficient oxygen and nutrients reaching the heart, causing the heart tissue to die or be at very high risk of death.
STEMI and NSTEMI are the 2 subtypes of myocardial infarction (MI), commonly known as a heart attack. In both types of MI, there is death (or infarction) of heart cells.
There are also two types of angina. Unstable angina is an acute condition where cell death has not yet occurred, but is at very high risk of occurring. In contrast, stable angina is not an acute coronary syndrome, but rather a chronic condition that is often exacerbated by exertion and relieved by rest and/or treatment.
ACS can result in immediate death if damage is severe enough. Patients who survive can be left with significant loss of heart function. However, if diagnosis and intervention are prompt and effective, lasting damage can be minimal.
Pathophysiology
Acute coronary syndromes occur when the coronary vessels are sufficiently occluded to cause tissue ischemia or infarction (death). More than 90% of ACS results from disruption of an atherosclerotic, partially stenosed plaque. The other 10% of cases are due to intense and prolonged vasospasm, often related to cocaine abuse. The three different types of ACS are differentiated by the degree to which they cause myocardial damage.
Vessel Occlusion
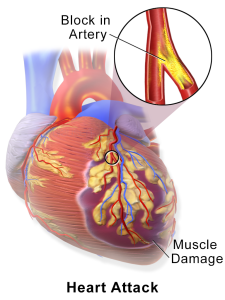
courtesy of Blausen Medical Communications
Inflammation is very important throughout the process of atherosclerosis. T cells produce TNF, IL-6, and IFN-gamma, stimulating endothelial cells and activating macrophages. Macrophage secretion of metalloproteinases weakens the fibrous cap and can lead to rupture. This can lead to acute inflammation, endothelial damage, platelet aggregation and thrombus formation. Atherosclerotic plaques of different sizes may become unstable as a result of these processes and undergo any of the following:
- rupture/fissuring, exposing highly thrombogenic plaque constituents
- erosion or ulceration, exposing thrombogenic basement membrane
- hemorrhage into the plaque, expanding its volume
These processes cause the vessel lumen to become blocked within minutes and cause a partial or complete occlusion of the vessel, which leads to varying degrees of ischemia.
In a STEMI: A thrombus forms that causes complete occlusion of the entire coronary artery, causing cell death.
In both UA/NSTEMI: There is a significant but incomplete blockage by the thrombus combined with an imbalance between oxygen supply and demand. The distinguishing feature of an NSTEMI vs UA is the presence of some degree cell death, indicated by cardiac biomarkers.
Supply and demand imbalances can occur because of adrenergic increases in blood pressure or local vasospasm increase demand on the heart. These can accompany awakening and arising, leading to the peak incidence of MI occurring between 6am-noon. Emotional stress can also contribute to plaque disruption, illustrated by the increase in sudden death following disasters. Vasoconstriction can also be mediated by platelet activation, and possible inflammatory mediators.
Given the pathologic processes described above, adults at high risk of ACS may be totally asymptomatic. It is also increasingly clear that plaque disruption and thrombosis can occur in silent repetitive waves.
Tissue Infarction
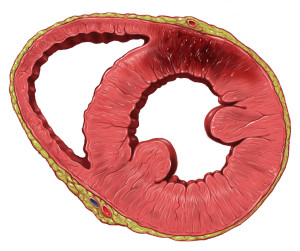
left ventricular infarction, courtesy of Patrick Lynch
Within seconds of vessel occlusion, aerobic glycolysis stops, and lactic acid begins to accumulate. A striking loss of contractility occurs within 60 seconds of ischemia onset. If ischemia continues, it causes an infarct and the cells in the affected area die and begin the process of myonecrosis. The heart undergoes coagulative necrosis, in which the cell structure is preserved for several days before beginning to degenerate.
A wave front of cell death moves through the wall, and necrosis appears largely complete within 6 hours. Hearts with developed coronary arterial collaterals may take up to 12 hours to fully necrose.
Once infarction stabilizes, the tissue begins to scar. Scarring is well advanced after 6 weeks. Ventricular remodeling includes progressive changes in size, shape, and thickness of the heart, leading to initial benefit but later impairment.
MIs can affect either the partial or complete thickness of the myocardial wall. Partial infarcts affect the subendocardium, which is the most susceptible area as it is the furthest away from the blood supply. Subendocardial infarcts can result from a thrombus which is lysed early after forming.
However, most MIs are transmural, in which necrosis extends through the entire thickness of the myocardium.
Vascular Territories
main article: cardiac blood supply
The heart has three major vessels – the left anterior descending, the left circumflex, and the right coronary artery. Each vessel nourishes a region of the heart, and disruption of each leads to specific effects on the heart’s function.
Nearly all transmural infarcts involve at least a portion of the left ventricle, including the septum. About 15-30% of those affecting the posterior wall extend into the right atrium. Atrial tissue can sometimes become infarcted with large posterior wall infarcts.
| Vessel | Areas supplied | Consequences | ECG leads |
| Left anterior descending |
|
|
ST elevation V1-V2 (septal) and V3-V4 (anterior) |
| Left circumflex |
|
ST elevation V5-V6, Reciprocal ST depression (V1-V3) | |
| Right coronary artery |
|
|
ST elevation II, III, aVF |
Causes and Risk Factors
Acute coronary syndromes typically result from a disruption of an atherosclerotic plaque in a blood vessel. Risk factors for atherosclerosis include:
- increased age
- male gender
- hypertension
- smoking
- diabetes
- hyperlipidemia
- family history and genetic polymorphisms
- infection and inflammation
- metabolic syndrome
- sedentary lifestyle
- substance abuse (particularly cocaine abuse)
Less common etiologies include any instance where there is significantly increased demand on the heart, coupled with poor myocardial perfusion (i.e. trauma with massive blood loss, anemia, infection, etc.).
Signs and Symptoms
Acute coronary syndrome is a medical emergency, and history and physical exam should be performed quickly but effectively, as ECG, bloodwork, and initial treatments are organized in parallel.
History
Symptoms of ACS vary considerably, and can be vague, especially in women or people with diabetes. They can begin or worsen with exertion.
Chest symptoms can include pain, heaviness, tightening, or pressure. Clinical characteristics that raise the likelihood of cardiac chest pain include:
- dull, pressure-like; retrosternal chest discomfort
- crushing pain
- duration approximately 2 to 5 minutes
- gradual onset
- reproducible/exacerbated with exertion
- pain not reproducible with palpation
In patients with pre-existing angina, ACS may be represented by a worsening of symptoms, ie frequency, severity, or duration, or may be unrelieved after 15 minutes of rest or nitro spray.
Pain can radiate to the:
- left arm or shoulder
- shoulder blades
- neck or jaw
- upper abdomen
Other symptoms can include:
- diaphoresis
- generalized weakness, fatigue, and dizziness
- dyspnea
- nausea, vomiting
- anxiety, or feelings of extreme unease
- new or unusual indigestion
In 10-15% of people, particularly the elderly and those with diabetes, silent MIs are only discovered later by ECG changes, usually new Q waves.
Identify relevant past medical history, including:
- previous coronary artery disease or stroke
- risk factors, including diabetes, hypertension, hyperlipidemia, and smoking
- family history of coronary artery disease
Physical Exam
When assessing a patient you suspect of having ACS, begin with an immediate and regular recording of the patient’s vital signs.
Perform thorough cardiovascular, respiratory, and abdominal exams. The goal is to explore for other factors that may contribute to the patient’s symptoms and increase or decrease the likelihood of a diagnosis of ACS.
Patients may have a rapid, weak pulse and are often sweating profusely.
Assess, in particular, for signs of heart failure. Look for evidence of right ventricular infarction, which include:
- jugular vein distension
- hypotension
- lung fields free
A useful way to remember factors that reduce the likelihood of chest pain being cardiac in origin is the “three P’s”:
- pleuritic
- positional
- reproducible by palpation
Investigations
The history and physical exam are crucial components of the assessment of any patient presenting with possible ACS. However, these should be obtained expediently as definitive diagnoses require electrocardiac studies and laboratory investigations.
Lab Investigations
Serum markers of myocardial injury are an important component of the ACS work-up. In an emergency department setting, these are most useful in patients with a non-diagnostic ECG, yet the clinician retains a strong clinical suspicion for myocardial injury.
Fatally injured myocytes leak proteins into the blood. These include myoglobin, troponins, creatine kinase-MB, lactate dehydrogenase, and others. The preferred biomarkers are cardiac-specific, particularly the troponins. An absence of change in CK within 2 days essentially excludes MI. Reperfusion accelerates biopmarker presence due to enzyme washout.
For appropriate interpretation, serial measurements are usually obtained every 6 – 8 hours until levels have peaked.
Cardiac markers include:
troponin-T -I, and -C
- much more sensitive
- begins to rise 3-4 hours, peaking at 12-36 hours
- remain elevated 7-10 days after event
creatine kinase-MB
- former gold standard
- begins to rise with 2-4 hours ,peaks at 24 hours
- returns to normal within 72 hours, much shorter than the troponins
myoglobin
- cleared quickly by kidneys
Perform a complete blood count, glucose level, and kidney testing to assess for general status. If abdominal causes of pain are being considered, liver and pancreatic testing may be done.
Before reperfusion: prothrombin time (PT) and partial thromboplastin time (PTT) to ensure extrinsic and intrinsic clotting pathways are ok
Imaging
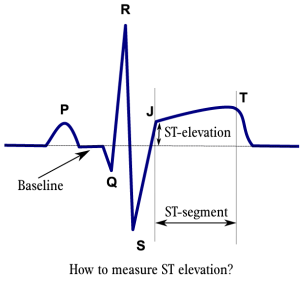
courtesy of Rob KreugerAn electrocardiogram (ECG) is an essential component of the patient work up, and should be performed within 10 minutes of medical contact.
Typical ECG findings of ACS include:
- ST segment elevations
- ST segment depressions (more likely ischemia) or elevations (more likely infarction)
- T wave morphology changes (hyperacute and inversion)
- QRS complex changes (new bundle branch block; evidence of Q waves – suggestive of old infarct)
- rhythm disturbances
The location of a myocardial infarct can be determined from the pattern of morphological changes on the ECG. ST changes are seen in patterns related to the cardiac territories, as described in this image.
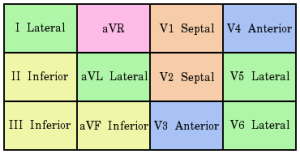
Contiguous leads on ECG, courtesy of Cburnett
If available, comparison with an old ECG is particularly helpful. It is important to note that ECG changes are dynamic and thus, change over time. Therefore, in a patient in which ACS is highly suspected, serial ECGs are often appropriate.
Chest radiography may reveal evidence of pulmonary edema, pneumonia, or aortic dissection.
Echocardiography is frequently used to evaluate patients with ACS. In the acute phase, use is limited, but may reveal regional wall motion abnormalities contribute to the evidence supporting a diagnosis of acute MI. It is more useful in assessing the extent of myocardial injury once the patient is stable.
Other diagnostic imaging modalities that may be used include myocardial scintigraphy and computed tomography.
Differential Diagnosis
The differential diagnosis of chest pain is extensive and includes both cardiac and non-cardiac causes; care should be taken to consider and rule out serious causes of chest pain, even while possible ACS is being evaluated.
cardiac
respiratory
|
gastrointestinal
other
|
Management
Out of hospital treatment
Acute coronary syndromes can be life threatening and should prompt immediate transport via EMS to hospital, if suspected. Frequently monitor vital signs, including heart rate, respiratory rate, blood pressure, and pulse oximetry if available.
Provide basic life support in the case of cardiovascular collapse.
Initial treatments, if available, may be remembered with the mnemonic MONA:
- Morphine
- Oxygen
- Nitroglycerin (contraindicated if a right-sided MI is suspected)
- ASA (if no allergy or recent/active GI bleed)
If available, a 12-lead ECG may be applied to assist with diagnosis.
In-Hospital treatment
Building on the steps described above, the following acute care may be provided in hospitals.
Immediate management
- IV fluid resuscitation if hypotensive, with vasopressor/inotropic support if warranted
- antiplatelet agents – both ASA and clopidogrel
- anticoagulants – low molecular weight heparin, or heparin
- nitrates – sublingually or IV (avoid with hypotension/R-sided ACS)
- beta blockers
- oxygen, in order to keep saturation above 92-94%
- morphine – reduces pain, oxygen requirement, preload, and afterload
Reperfusion
Fibrinolytics may be given in cases of STEMI, though not NSTEMI or UA. They should be given within 12 hours of onset of symptoms, though aim for a door to needle time below 30 minutes.
Absolute contraindications to fibrinolytic therapy include:
- prior intracranial bleed
- head or facial trauma within 3 months
- known cerebral vascular lesion
- intracranial malignancy
- ischemic stroke <3 months
- active bleeding
- suspected aortic dissection
Relative contraindications include:
- severe hypertension (180/110)
- current anticoagulation
- recent noncompressible vascular punctures
- major surgery within 6-8 weeks
- embolic stroke within 3-12 months
- pregnancy
- active peptic ulcers
Cardiac catheterization,s or percutaneous coronary intervention (PCI) should be considered in patients with STEMI, or NSTEMI and:
- recurrent angina, despite therapy
- CHF or LV dysfunction
- hemodynamic isntability
- high TIMI score
- recurrent or sustained ventricular tachycardia
- dynamic ECG changes
- high-risk findings
- previous PCI within last 6 months
- repeated episodes of acute coronary syndrome
Patients who are undergoing PCI should be pre-treated with anti-platelet therapy.
Contraindications to catheterization include:
- severe peripheral vascular disease (difficult catheterization)
- chronic renal insufficiency (relative contraindication)
Post-Acute Treatment
Patients should be monitored for stability in hospital regarding arrhythmias and cardiac function.
The following medications should be started in the post-acute phase:
- angiotensin-converting enzyme (ACE) inhibitors within 24 hours
- statins within 24 hours
Patients should remain on anticoagulants such as ASA or clopidogrel, with the length of treatment determined by interventions such as stenting during PCI.
Following discharge from hospital, patients should be followed up regularly. Review should include:
- patterns of chest pain
- need for nitro spray
- medication side effects
- adherence to medication plan
- other symptoms, including shortness of breath, palpitations, pre-syncope, or neurological symptoms.
Risk factor management is incredibly important, including:
- referral to a cardiac rehabilitation program to develop safe, longstanding exercise regimens
- adherence to evidence based lifestyle changes, such as the Mediterranean diet
- control of hypertension, diabetes, and, dyslipidemia
- support for smoking cessation
Complications
Nearly 75% of people have one or more complications after ACS, which are categorized as ischemic, mechanical, or arrhythmic.
Ischemic: Even after an event, there may be ongoing ischemia if the blockage returns, if the plaque continues to grow, or if there is significant disease in other arteries. If the thrombus continues to grow, there can also be infarct extension or expansion. Continued ischemia is a common finding in MIs and can lead to further damage even if no symptoms are present.
Mechanical: Necrosis of the myocardium causes both a failure of the cardiac myocytes to function and weakening of the heart structure. This can make the heart susceptible to other mechanical events, including:
- Ventricular septal rupture – creating a ventricular septal defect, which increases pressures on the right side of the heart
- Ventricular aneurysm – can lead to blood stasis, thrombus development, and cardiogenic shock
- Ventricular pseudoaneurysm – ballooning into pericardium creates higher risk for rupture
- Free wall rupture
- Papillary muscle rupture – causing mitral or tricuspid regurgitation
- Left/right ventricular failure – leading to cardiogenic shock
Arrhythmic:
Reperfusion is the best way to salvage ischemic heart tissue, but can lead to arrhythmias, contraction bands (supercontraction of already dead cells), or reperfusion injury, mediated, at least in part, by free radicals. Some common arrhythmia that occur include:
- Fast: V-tach, V-fib, A-fib, sinus tachycardia, idioventricular rhythms
- Slow: Sinus bradycardia
- Aberrant: Bundle branch blocks, AV blocks
Sustained arrhythmias may be treated with antiarrhythmics (main article antiarrhythmic medications). Given high morbidity accompanying post-ACS arrhythmias, these treatments should be administered under expert supervision.
Aside from the physiological consequences, many people can also experience psychosocial challenges following an ACS, including:
- depression and anxiety
- changes in employment or social activities
Prognosis
In-hospital mortality has declined from 30% in the 1960’s to 10-13% today (ref). Half of deaths occur within 1 hour of symptom onset.
Both short and long term survival rates are compromised for those who have experienced an ACS. In Canada, mortality rates are 3x greater for MI survivors at 1 year as compared to the average population.
Of those who have a second incident, 50% occur within the 1st year. This increased risk is associated with continued progression of arterial disease, highlighting the importance of post-ACS care.
Resources and References
Andreoli et al. Cecil Essentials of Medicine 7th Ed. Saunders Elsevier. Philadelphia 2007.
Marx et al. Rosen’s Emergency Medicine: Concepts and Clinical Practice 7th Ed. Mosby Elsevier. Philadelphia 2010.
Porter et al. The Merck Manual of Patient Symptoms: A Concise, Practical Guide to Etiology, Evaluation, and Treatment. Merck & Co., Inc. New Jersey 2008.
Smolina K, Wright FL, Rayner M, Goldacre MJ. Long-Term Survival and Recurrence After Acute Myocardial Infarction in England, 2004 to 2010. Circ Cardiovasc Qual Outcomes. 2012 Jul 1;5(4):532–40.
Tintanalli, JE Emergency Medicine (A Comprehensive Study Guide). McGraw-Hill Companies, Inc. USA 2004.
Unstable angina: MedlinePlus Medical Encyclopedia [Internet]. [cited 2014 Jul 16]. Available from: http://www.nlm.nih.gov/medlineplus/ency/article/000201.htm
Part 3: Adult Basic Life Support. Circulation. 2000 Aug 22;102(suppl 1):I–22–I–59.
Babu KS, Salvi SS. Aspirin and asthma. Chest. 2000 Nov 1;118(5):1470–6.
