last authored: May 2016, David LaPierre
last reviewed: May 2016, Colin McCabe
Introduction
The heart rhythmically contracts at a rate of between 60-100 beats per minute (bpm) at rest to maintain effective cardiac output. Effective control of heart rate is of paramount importance to normal function, and heart rate abnormalities – too slow, too fast, or poor coordination – can rapidly prove to be lethal if of a great enough severity.
The coordinated cardiac cycle is due to a sophisticated conduction system that distributes impulses throughout the heart.
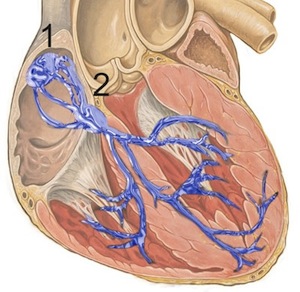
1: sinoatrial node; 2: atrioventricular node used with permission, J Heuser
The heart has an independent pacemaker system that can function without external control. This normally initiates at the sinoatrial node (1 in the diagram) in the right atrium. Electrical conductance travels across the atrium to the atrioventricular node (2 in the diagram) where it then branches to innervate the left and right ventricles through the bundles of His.
External control of heart rate, through both neurological and endocrine involvement, allows cardiac output to respond to increased metabolic demand during times of exertion or stress.
Specialized action potentials, which differ amongst different cardiac cells, are responsible for electrical propagation. Cardiomyocytes themselves have action potentials of their own, described further below.
The Sinoatrial Node
Located in the posterior wall of the right atrium, near the junction of the superior vena cava, the SinoAtrial (SA) node is the physiologic pacemaker of the heart, with an intrinsic firing rate of 60-100 impulses/min. It is a small mass of cells which emit regular electrical impulses across both atria, like ripples in a pond. Cellular structures called intercalated discs and gap junctions among cardiomyocytes allow direct flow of the action potential from cell to cell until the it reaches the AV node.
The Atrioventricular Node and Ventricular Innervation
Internodal pathways lead across the atrium to the atrioventricular (AV) node. Fibrous tissue surrounds the tricuspid and mitral valves, normally preventing signals from traveling between the atria and ventricles other than through the AV node.
Fibres here are smaller in diameter, and the impulse is thereby slowed with a delay of 0.1 sec to allow time for the atria to contract and empty before ventricular systole. As well, during abnormal atrial rhythms (eg atrial fibrillation), this delay regulates ventricular stimulation.
If the SA node or atria are not functioning properly, the AV node can act as a pacemaker, firing at 40-60 impulses/min.
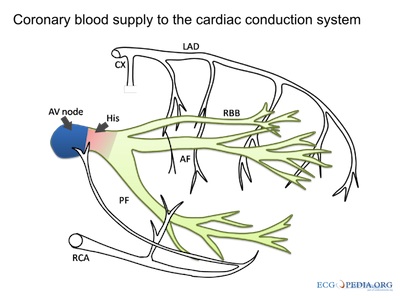
courtesy of ECGpedia.org
The Bundle of His (AV bundle) leaves the AV Node and splits into the right and left bundle branches, which innervate the ventricles via Purkinje Fibres. Purkinje fibres spread along the endocardium, and the lower part of the ventricles contract before the upper parts. In the absence of both nodal pacemakers, the Bundle of His and Purkinje Fibres can fire at 15-40 impulses/min. This rate, a form of bradycardia, is normally unable to sustain life indefinitely.
Cardiac Action Potentials
Action potentials (APs) are the electrical propagation signals through individual cells and are mediated by complex ion flow through specialized channels. Cardiac myocytes maintain an electrical gradient across their membrane, with the inside more negative. The resting potential of cells is normally -60 mV, and is regulated by the distribution of ions inside and outside the cell. Sodium and calcium concentrations are much higher outside than inside, while potassium concentrations are higher inside the cell. Ions flow down their ion gradient, from areas of high concentration to low concentration.
The resting potential is maintained by an array of channels and pumps for sodium, potassium, and calcium. Of critical importance is the Na/K ATPase, which pumps three Na ions from inside the cell and exchanges these for two K ions from the outside. Another important channel is the IK channel.
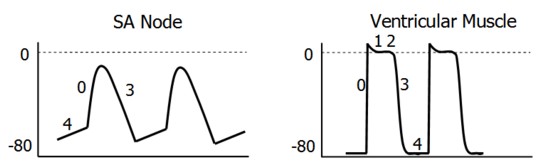
| 0 | ICa inwards |
| 3 | IK outwards |
| 4 | If (Na) inwards |
SA/AV Node flow
Pacemaker cells have a spontaneous tendency to depolarization, or automaticity, mediated by a pacemaker current, thought to be predominantly via sodium influx through channels which open during repolarization. As the cell depolarizes, voltage-gated calcium channels open to allow calcium to flow down its gradient, into the cell. This flow results in the action potential being initiated. With depolarization, the calcium channels close and potassium channels open. Potassium floods out of the cell, down its gradient, and the cell is repolarized, to begin the cycle again.
Differences in pacemaker rate depend on rate of sodium flow, maximum negative diastolic potential, and threshold potential. The cell group with the fastest intrinsic rhythm prevents all other automaticity using overdrive suppression. This is due to hyperpolarization which occurs when a cell is forced to fire faster than its pacemaker rate. Molecularly, increased sodium influx requires faster pumping out, resulting in greater intracellular potassium (via the Na/K pump).
The bundle of His and Purkinje cells, with their very high concentration of sodium channels, rapidly conduct depolarization signals from the AV node across the ventricles.
Cardiomyocyte Action Potentials
Unlike the pacemaker cells of the heart, cardiomyocytes in the atria and ventricles do not spontaneously depolarize. Instead, they rely on signals from the electrical components of the heart – the SA node, AV node, bundle of His, and Purkinje fibres.
| 0 | ICa, INa inwards |
| 1 | INa ends, IK outwards |
| 2 | ICa inwards |
| 3 | ICa ends, IKoutwards |
| 4 | maintenance |
Phase 0: Once depolarization signals arrive at cardiomyocytes, sodium channels open. Once threshold is reached (-70 mV), a self-sustaining Na inward current carries the cell to 0 mV and transiently into the positive range.
Rapid inactivation of Na channels makes sodium flow short-lived. Calcium L-type channels open at -40mV and remain open for a much longer period, providing the signal for contraction.
Phase 1: Following depolarization, potassium channels transiently open to return the membrane potential to 0 mV.
Phase 2: An outward K+ current is balanced by the still-happening inward Ca2+ current, leading to the plateau.
Phase 3: The gradual inactivation of Ca2+ channels, and the continued outward K+ current, returns the membrane potential to -90 mV. A long refractory period allows ventricles sufficient time to eject blood and refill before the next contraction.
The degree of refraction depends on the number of inactivated sodium channels. The absolute refractory period is followed by the relative refractory period, during which greater than normal stimulation is required to depolarize the cell. The atrial refractory period is generally shorter than that of ventricular cells, meaning atrial rates can exceed those of the ventricles during arrhythmias.
Phase 4: Open K+ channels keep cardiomyocytes stable at -90 mV, as predicted by the Nernst Equation. The cell is thus ready for the next round of depolarization.
To maintain ion gradients, various pumps remove ions to the extracellular space and return calcium to the sarcolemma.
Control of Heart Rate
Heart rate is largely controlled by the autonomic nervous system, originating in the brainstem medulla. The autonomic nervous system has two divisions – the sympathetic nervous system (SNS) prepares the body for “fight or flight” and the parasympathetic nervous system (PNS) prepares the body to “rest and digest”. As one would expect, the sympathetic nervous system increases cardiac output, both by increasing heart rate and contractility. Parasympathetic innervation, in contrast, slows the heart rate down.
Increasing heart rate
The sympathetic system leaves the spinal cord at each vertebral level. Innervation of the heart originates from thoracic nerve roots T1-T4 (sometimes T5). Norepinephrine is released by nerves that innervate the heart to directly impact cardiac function.
Hormonal control of heart rate is primarily mediated by the release of epinephrine (also known as adrenalin) from the adrenal gland. Release is normally in response to “fight or flight” situations, mediated by sympathetic innervation of the adrenal gland, and works across the body. Epinephrine’s effects on increasing heart rate are very similar to the effects of norepinephrine.
Both epinephrine and norepinephrine work on beta-1 adrenergic receptors to increase the heart rate and contractility (stroke volume). These signals work to:
- Increase pacemaker rates of the SA and AV nodes and Purkinje Fibres. This occurs through by increases in ICA2+ and INA+ channel openings, speeding depolarization and reaching threshold faster
- Increase conduction velocity of the SA and AV nodes This occurs through increased IF channel opening
Decreasing heart rate
The parasympathetic system works to decrease heart rate through the vagus nerve, which originates directly from the brain stem and travels across the body, including to the heart. The vagus nerve releases the neurotransmitter acetylcholine.
Acetylcholine decreases heart rate in the opposite fashion as (nor)-epinephrine. It works to:
- Decrease pacemaker rates of the SA and AV nodes and Purkinje Fibres, by increasing IK+ permeability, hyperpolarizing the cells, and by decreases ICA2+ and INA+ channel openings, depolarization is slowed down, reaches threshold slower
- Decrease conduction velocity of the SA and AV nodes, by decreases IF channel opening
Thyroid Gland
The thyroid gland plays a major role in mediating metabolic function. Thyroid hormone (TH) affects the heart rate over time by mediating synthesis of beta adrenergic receptor which causes an increase in heart rate. Excessive TH, as can take place with hyperthyroidism, can therefore lead to tachycardia (rapid heart rate). Insufficient TH, or hypothyroidism, can lead to bradycardia (slowed heart rate).
Resources and References
Khan Academy – heart depolarization
Topic Development
authors: Raksha Sule (2012), David LaPierre
Reviewers: Colin McCabe
